RESEARCH TEAMS
IMMUNE RESPONSES IN THE IMMUNOCOMPROMISED HOST: TOLERANCE VERSUS GVHD
Our projects address the pathophysiological mechanisms of immune responses in immunocompromised subjects, mainly in the context of allogeneic hematopoietic stem cell transplantation (allo-HSCT) but also in other settings such as HHV8-related diseases. Besides, we also consider new therapeutic approaches for manipulating immune responses. These projects combine fundamental, preclinical, and clinical studies.
Allo-HSCT is a major treatment for hematopoietic malignancies. Treatment efficacy is mainly due to the anti-tumoral responses induced by donor T cells (graft versus leukemia effect, GVL). However, graft-versus-host disease (GVHD) occurs when donor T cells recognize and target healthy tissues in recipients. Other complications include opportunistic infections or reactivation of latent viral infections, as long as the immune system is not fully recovered. Our team covers various projects at the crossroads of hematology, immunology and virology, mostly aiming at unraveling pathophysiological mechanisms involved in post-transplant complications. Of note, the team has particular interest in non-conventional lymphocytes, mainly mucosal-associated invariant T cells (MAITs), but also invariant natural killer T cells (iNKT), innate lymphoid cells (ILCs) and various immunoregulatory subsets (Tregs, Tr1…).
Together, all team members share long-lasting interest and expertise in the field of translational and basic immunology focusing on immune responses in the immune compromised host, especially on GVL and GVHD. We have developed strong scientific and clinical collaborations taking the advantage of the geographic vicinity with the closely associated Hematopoietic stem cell transplantation, Immunology and Virology Departments at St-Louis Hospital, strengthening our translational programs.
Figure: U976 Team 4 / Main projects:
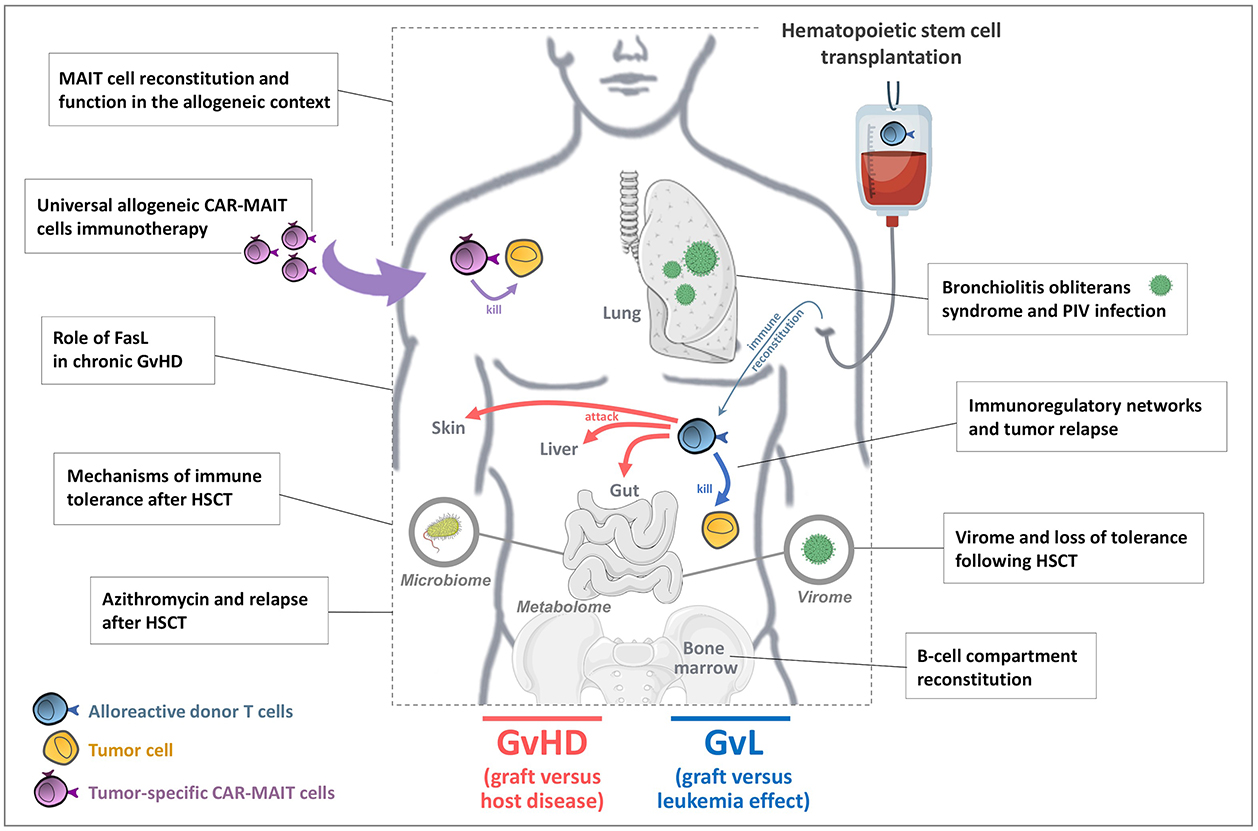
PROJECTS
MAIT cell functions and usage in the allogeneic context
Project managers: Sophie CAILLAT-ZUCMAN
We investigate the reconstitution dynamics and functional role(s) of mucosal-associated invariant T cells (MAITs) after HSCT, and also consider their use as a source of universal cells to produce chimeric antigen receptor (CAR-MAIT) cells.
Mucosal-associated invariant T cells (MAITs) are non-conventional semi-invariant T lymphocytes. Upon recognition of microbial riboflavin derivatives presented by the non-classical MHC class I-related MR1 protein, MAITs secrete Th1/Th17 cytokines and cytotoxic molecules, and contribute to rapid protection against microbes. MAITs have also recently been given regulatory and tissue repair functions.
We investigate the role of MAITs after HSCT, i.e. their reconstitution dynamics, spatial relationship with mucosa and functional profile, in relation with clinical events (GVHD). Because their TCR is of very limited diversity, it is unlikely that MAITs can directly recognize allogeneic cells. However, they can also be activated by combination of particular cytokines. Depending on the inflammatory milieu, MAITs might have regulatory rather than antimicrobial functions, via secretion of soluble factors involved in gut epithelial barrier integrity and shown to play a major role in experimental GVHD. We are thus exploring the potential usage of MAITs in adoptive therapy to treat or prevent GVHD.
We are also considering MAITs as a source of universal cells to produce chimeric antigen receptor (CAR-MAIT) cells for the treatment of hematologic malignancies in the allogeneic setting. To date, CAR-T cells are usually produced for each patient (autologous CAR-T), which is prohibitively expensive and logistically challenging, leading to the development of alternative strategies. We are generating CD19 CAR-MAIT cells as proof-of-concept, and assess their engraftment capacity, tumor killing efficacy and off-target toxicity in preclinical mouse models. Such universal, off-the-shelf, product generated from third-party healthy donors would facilitate standard-of-care requirements and offset the costs of individual cell preparation.
Tolerance following HSCT
Mechanisms of immune tolerance after HSCT using global approaches
Project managers: Gérard SOCIÉ, David, David MICHONNEAU
With the aim of better understanding GVHD physiopathology, we investigate various parameters potentially involved in GVHD versus operational immune tolerance, using deep phenotyping, metabolomics and transcriptomics approaches. We use mass cytometry and RNA sequencing to characterize most relevant immune populations with regard to acute or chronic GVHD, and tolerance versus non tolerance. We also analyze circulating metabolites from main biochemical pathways in tolerant and non-tolerant recipients and their donors. This longitudinal project will provide an integrated view of GVHD pathophysiology and mechanisms of immune tolerance in humans. Identification of cellular, transcriptomic or metabolomic profile associated with GVHD or tolerance in donors and/or recipients will be integrated using integrative bioinformatics tools.
Protective role of FasL in cGvHD
Project managers: Saoussen KARRAY
We have developed in the past years an experimental model to study chronic GVHD, and more particularly the role of FasL in this setting. We found that FasL deficiency on alloreactive donor cells significantly reduces GvHD scores and ameliorates mice survival. We are currently investigating the role of FasL specifically in allogeneic T or myeloid cells to better understand cell types and mechanisms that might be implicated in this protection.
B-cell compartment reconstitution
Project managers: Gérard SOCIÉ
Following allo-HSCT, patients show humoral immunity deficiency until 1 year post-transplant. We investigate the mechanisms responsible for the delayed recovery of humoral immune responses after allo-HSCT. Specifically, we aim to (i) provide an exhaustive characterization of B cell reconstitution in transplanted patients; (ii) follow the kinetics of immunoglobulin heavy chain (IgH) repertoire reconstitution; (iii) refine current developmental staging of human bone marrow B cell precursors and screen new regulators of B cell precursor survival, proliferation and maturation, and (iv) develop biostatistics tools to integrate clinical data, and analyze high throughput gene expression profiles and antibody repertoire datasets. This should help to better define the immune status of transplant recipients, orient their therapeutic management and lay the foundations for novel therapeutic interventions.
Post-transplantation tumor relapse
Immunoregulatory networks and graft-versus-leukemia effect
Project managers: Mathieu CHEVALIER
Although allo-HSCT is the best treatment for intermediate/high risk acute myeloid leukemia (AML), tumor relapse still occurs in around 40% of patients. In various malignant diseases, several immunoregulatory mechanisms have been shown to restrain constitutive and treatment-induced effector immune responses. These fine-tuned mechanisms include ligand-mediated engagement of inhibitory receptors, and induction of immunosuppressive cell-subsets. We aim to provide a comprehensive understanding of the immunoregulatory mechanisms associated with tumor relapse in AML patients receiving HSCT. This should not only provide fundamental insights into the immunoregulatory landscape reconstituting following HSCT, but may also help identify prognostic tools and/or therapeutic targets able to restore subverted anti-tumor immunity.
Anti-tumor immune response after azithromycin treatment
Project managers: David MICHONNEAU
Recently, a double-blind, randomized multicentre clinical trial (ALLOZITHRO) evaluated the effect of the azithromycin antibiotic (AZM) on airflow decline-free survival after allo-HSCT. It was prematurely discontinued due to an increased incidence of relapse in the AZM arm, without any impact on GVHD incidence. We are investigating mechanisms underlying relapses in patients treated with AZM. Our study aims at understanding how immune and microbial landscape are altered by azithromycin and the relationship that interconnects host microbiota, metabolism and immune system. Deciphering the role of AZM in post-transplantation hematologic relapses, and more generally in antitumor response and tumor cell biology, may have a major clinical impact to understand risks associated with AZM intake.
Virus infections in immunocompromised hosts
Gut Virome and GVHD
Project managers: Jérôme LE-GOFF
We recently characterized the gut virome, microbiome and phagosome from HSCT recipients using metagenomics. Eukaryotic viruses, that usually represent a minor population in the gut compared to phages, accounted for more than 30% of viral sequences early post-transplant. Our data highlighted the wide diversity and evolution of eukaryotic viruses after HSCT and unveiled an unexpected association of Picobirnaviruses (PBV) with post-transplant allogeneic reactivity, paving the way of studying the mechanisms underlying virus-induced loss of tolerance.
Among enteric viruses, reactivations of Adenoviruses occur frequently after HSCT, especially in children, and are closely associated with digestive GVHD. Adenovirus infections are associated with a high rate of morbidity and mortality. As there is no drug currently available for the treatment, we investigate novel antiviral strategies.
Respiratory virus infections after HSCT
Project managers: Jérôme LE-GOFF
Respiratory viruses are associated with non-infectious pulmonary complications of HSCT, such as the bronchiolitis obliterans syndrome (BOS). Bronchial epithelium of HSCT recipients presents phenotypic characteristics associated with viral — in particular paramyxovirus — infections triggering BOS. We are developing an ex vivo model of bronchial mucosa from biopsies obtained from HSCT recipients and controls. We investigate mucosal and immune responses to parainfluenza virus (PIV) type 3 infection using transcriptomic and phenotypic analyses. This project should help better understanding pulmonary complications following HSCT.
Immunology of HHV8-related diseases
Project managers: Guislaine CARCELAIN, David BOUTBOUL
The team also focuses on HHV8-related Castleman disease, a life-threatening lymphoproliferative disorder mainly occurring in immunocompromised hosts. This activity is closely related to the clinical activity of the French National Center for Castleman disease led by Pr E. Oksenhendler, with more than 200 patients included in this large cohort. This program has been set up in collaboration with Pr J. Le Goff and is aiming to develop new and rapid diagnostic tools but also to study the immune mechanisms involved in the development of the disease (host and viral B-cell reprograming, T-cell responses).
Principal Investigators
- Pr Sophie CAILLAT-ZUCMAN : Team Leader
- Pr Gérard SOCIÉ : Co-director
- Pr Jérôme LE-GOFF
- Dr David MICHONNEAU
- Dr David BOUTBOUL
- Pr Guislaine CARCELAIN
- Dr Mathieu CHEVALIER

Latest publications
Circulating T cell profiles associate with enterotype signatures underlying hematological malignancy relapses.
Jun 10, 2023
Milestones in acute GVHD pathophysiology.
Dec 5, 2022


