RESEARCH TEAMS
ENDOTHELIUM, INFLAMMATION & ALLOGENICITY
The endothelium has multiple functions in immune homeostasis and alterations of endothelial function can perturb immune function either by becoming a target of immune responses resulting in endothelial damage or by initiating aberrant immune responses.
This team studies the implication of human microvascular endothelium in both innate and adaptive immune responses and particularly in the inflammatory environments of organ transplantation and sepsis.
PROJECT
Chronic antibody-mediated rejection (AMR) of solid organ transplants is characterized by activation and injury of the allograft endothelium. The allograft endothelium is the first site of interaction between the donor organ and the recipient; the endothelium is thereby exposed to the alloimmune response. Most alloantibodies are directed against donor HLA antigens and named « Donor-specific-antibodies « (DSA). Circulating DSA are associated with endothelial damage and an inflammatory infiltrate of the allograft.
In an inflammatory setting, the microvascular endothelium increases HLA II antigen expression leading to allo- CD4+-T activation and differentiation towards Treg (CD54-dependent) and Th17 (IL-6-dependent) (Taflin et al 2011, PNAS). Later studies revealed that HLA II-specific antibody binding to endothelial cells further increased IL-6 production in an allogeneic setting, reinforced Th17 differentiation and led to a relative loss of Treg (Lion et al 2016, Am J Transplant). Exposure to commonly used immunosuppressors demonstrated differentially altered endothelial immunogenicity.(Lion et al 2017, Fr Immunol.).
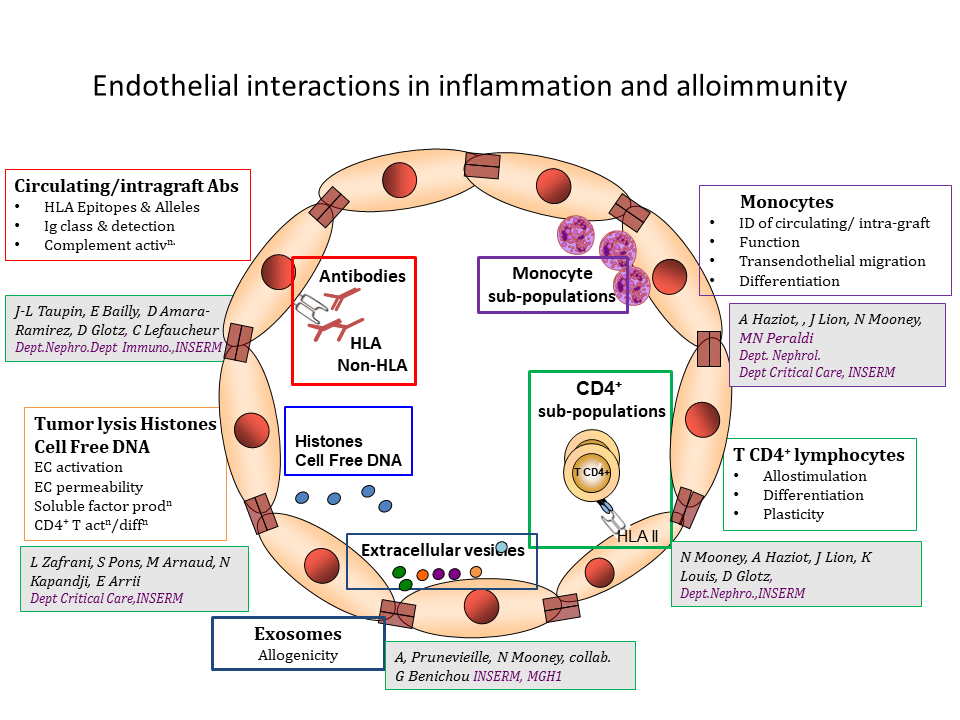
Although HLA-DR is the prototypical HLA II antigen, endothelial expression of HLA-DQ is both temporally and quantitatively different to HLA-DR. Post-transplant, HLA-DQ-specific DSA are prevalent. We have modeled DSA binding to HLA-DQ and reported a selective loss of endothelial ability for Treg differentiation as well as cooperation between HLA-DR and -DQ alloantibodies (Cross et al 2019, Kidney Int ; 2018, Fr Immunol.).
Intragraft infiltration by myeloid cells is a hallmark of Antibody Mediated Rejection. Extensive phenotypic and functional characterization of circulating monocytes was carried out in healthy individuals. The results of this analysis revealed an unknown and extensive diversity of circulating monocytes (Mourah-Merah et al,2020, Sci Reports). These populations have not been examined in the inflammatory setting of organ transplantation.
Tumour lysis syndrome (TLS) is a complication of cancer treatment in patients with extensive, rapidly growing, chemosensitive malignancies. It mainly occurs as a consequence of diverse anticancer treatments and is most frequent in cancers with high potential for cell lysis including acute leukemias, high-grade lymphomas, and other rapidly proliferating tumors. Histological analysis of TLS in mouse models disclosed renal endothelial damage and microthromboemboli composed of chromatin, cellular debris, fibrin, platelets, and malignant cells that occluded the renal capillary vessels. When cell death is extensive, high levels of unchained extracellular histones are released (Xu et al Nat Med 2009). These observations led us to study the outcome of histone binding to endothelial cells in in vitro and in vivo models. Histone-mediated activation of the endothelium by innate receptors was detected resulting in phenotypic and functional changes associated with altered endothelial immunogenicity (in vitro) and disruption of endothelial barrier function (in vivo) (Zafrani et al 2020 In prep.).
Principal Investigators
- Nuala MOONEY (DR1)
- Denis GLOTZ (PU-PH)
- Alain HAZIOT (DR)

Latest publications
Separating the Wheat from the Chaff among HLA-DQ
Apr 22, 2024



