RESEARCH TEAMS
ONCO-DERMATOLOGY AND THERAPIES
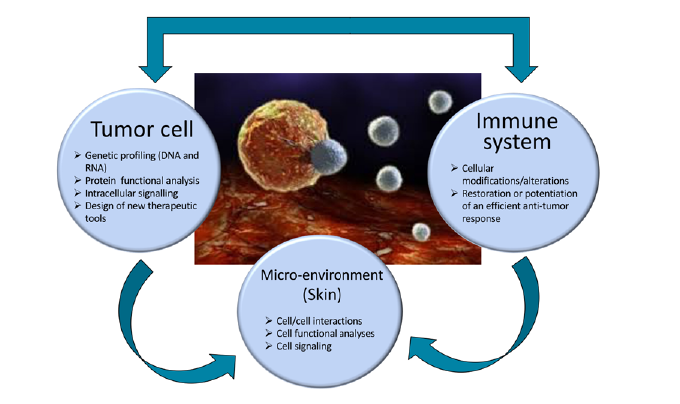
Our team has expertise in skin cancers, with a special interest in cutaneous T cell lymphoma, melanoma and carcinoma. We investigate the cellular and molecular mechanisms involve in cellular transformation and tumor escape, with a special focus on pathways leading to resistance or relapse upon therapies and inhibition of anti-tumor immune responses. Our research shows a strong “bench-to-bedside” aspect, combining the clinical study of new medical therapeutics with laboratory research on relevant tumor and immune targets.
Our team develops research projects dedicated to skin cancers with a main interest on melanoma, cutaneous T cell lymphoma (CTCL) and carcinoma. Although these diseases arise from the transformation of different cell types (namely melanocytes, CD4+ T cell and epithelial cells), they share common features such as a partially elucidated pathophysiology, a lack of treatment at advanced stages, a need to understand the mechanisms leading to relapse or resistance upon currently available treatments and consequently a need for alternative therapies.
In an effort to gain insights into these unresolved issues, we adopted research strategies to understand the mechanisms leading to:
- the promotion of tumor cells growth and spreading (identification of new oncogenes and tumor antigens, and of their related signalling pathways),
- an inhibition of the anti-tumor immune responses (modification of the micro-environment and neo-synthesis of inhibitory molecules by the tumor),
- the development of strategies by tumor cells to adapt and escape to antibody or protein inhibitors-based therapies.
Combined to our long-lasting and strong collaboration with the Onco-Dermatology Department of Saint Louis Hospital, our results would ultimately lead to the identification of novel targets both on the tumor and immune side and allow the development of innovative therapies.
PROJECTS
Cutaneous T cell lymphoma : cellular characterization and development of novel therapies
Project managers: A. Marie-Cardine, M. Bagot, M. Battistella, A. Bensussan
Mycosis Fungoides and Sézary syndrome are two forms of cutaneous T cell lymphoma associated to a poor prognosis at advanced stages due to a lack of curative treatments. Our main goal is to identify new tumor antigens specifically expressed at the tumor cell surface and to validate their potential use as new therapeutic targets through the development of antibody-based immune-therapies.
Cutaneous T cell lymphoma (CTCL) is a heterogeneous group of non-Hodgkin lymphomas that originate from the skin. We mainly focus our research activity on two types of CTCL: the Mycosis Fungoides (MF), where tumor cells accumulate in the skin, and the Sézary syndrome (SS), a highly aggressive form characterized by the presence of malignant cells in the skin and blood. Notably, the pathophysiology of these diseases is still controversial especially regarding the tumor cell origin or the tumorigenic triggering factors. Although malignant cells were identified as a clonal expansion of CD4+ T cell with memory phenotype, their phenotypic detection relies on the loss of pan-T cell antigens such as CD26 or/and CD7. Our previous work allowed the identification of the first positive marker for MF and SS cutaneous and circulating malignant T cells, namely the NK cell receptor KIR3DL2. By performing ex vivo experiments on circulating cells from Sézary patients, we established that KIR3DL2 was functional on tumor cells and acted as an inhibitory co-receptor. We further actively participated, in collaboration with the Biotech company Innate Pharma, to the selection and characterization of an anti-KIR3DL2 therapeutic antibody. Our in vitro, ex vivo and in vivo results demonstrated that such antibody was able to promote depletion of the malignant cells by antibody-dependent cell-cytotoxicity or -phagocytosis. The proof of concept being established, a phase I/II clinical trial was set that showed convincing results in terms of therapeutic safety and efficacy on Sézary patients.
Because 10% of Sézary patients display malignant T cells that do not express KIR3DL2, we pursue our search for specific tumor antigens. We recently reported that CD39, an ectonuclotidase involved in the process of generating adenosine from ATP, is aberrantly expressed by Sézary patients’ malignant cells. The adenosine pathway was identified as a key checkpoint inhibitory pathway able to negatively regulate immune responses, we therefore currently investigate the function of CD39, and its partner CD73, in the context of Sézary syndrome to evaluate their involvement in the generation of an adenosine-rich microenvironment leading to anti-tumor immune responses inhibition. This study would validate the use of anti-CD39 or anti-CD73 antagonist antibody as a pivotal tool for the development of new therapeutic approaches in the context of CTCL.
Melanoma – PDE4 and RICTOR : new targets in melanoma?
Project managers: N. Dumaz, C. Lebbé
The group gathers researchers with expertise in different aspects of melanoma biology. Major themes are centered on melanoma were developed with a common objective to discover new therapeutic targets for melanoma treatment and novel biomarkers of melanoma susceptibility.
The projects we developed on melanoma are centered on the study of signal transduction downstream of the main oncogenes mutated in melanoma with the aim of discovering new therapeutic targets for these tumors. We work in close collaboration with the onco-dermatology department to rapidly transfer ours results to the clinics through translational studies and clinical trials.
We have recently shown that RICTOR, a protein belonging to the mTORC2 complex, plays a major role in the control of the PI3K pathway in melanocytes and melanomas. We have demonstrated that the RICTOR locus is frequently amplified in melanomas and that the overexpression of RICTOR in melanocytes transformed by the NRAS oncogene stimulates their clonogenicity. We are now exploring in more detail the role of RICTOR amplification in melanocytes and melanomas. In particular, we have shown that although BRAF and RICTOR independently regulate two distinct signaling pathways (MAPK and PI3K, respectively), the BRAF protein interacts with the RICTOR protein in melanomas. We are studying the role of this interaction in detail. In addition to amplifications of the RICTOR locus, mutations of RICTOR were found in 8% of melanomas. We will determine the effect of these mutations on mTORC2 complex activity, AKT activation and melanocyte transformation by NRAS and BRAF. We have observed that the overexpression of RICTOR in melanocytes transformed by the NRAS oncogene increased the expression of the cyclin D1. We will determine by which mechanism RICTOR regulates the expression of the cyclin D1. These results will allow us to better understand the role of RICTOR (wild-type and mutant) in the transformation of melanocytes determine the role of the interaction of RICTOR with BRAF and its therapeutic potential.
We have shown that inhibition of the cAMP pathway by phosphodiesterase type 4 (PDE4) is necessary for the transformation of melanocytes by BRAF and RAS oncogenes and that PDE4D is overexpressed in metastatic melanomas and regulates invasion through its interaction with FAK. PDE4D is a major regulator of the cAMP pathway and is starting to emerge as an important actor in several cancers where its expression is often associated with bad prognosis. We are planning to analyze in greater detail the effects of PDE4 inhibitors ex vivo and in vivo and understand the regulation mechanisms of PDE4D in melanomas. We will test the effect of PDE4 inhibitor, in particular in combination with the inhibitors of the MAPK pathway, which are already used in clinics, in mouse xenograft models, and PDX models that we recently developed. In parallel, we will study the regulation of the expression of PDE4D in melanomas. Our data in situ demonstrated that the overexpression of PDE4D was done at the level of the mRNA and in particular through the overexpression of the isoform PDE4D5. We will investigate which signaling pathways regulate the transcription of PDE4D5 in melanomas and which transcription factors bind to the PDE4D5 promoter that has already been identified.
Melanoma – Functional and molecular characterization of CD147/EMMPRIN and Kindlin-3, two related molecules, involved in tumor microenvironment modulation
Protein/protein interactions in the control of apoptosis - Development of new therapeutic tools
Project managers: J-L. Poyet
Cancer is a major public health issue and a tremendous challenge to drug discovery relative to identifying key therapeutic targets as well as developing breakthrough medicines. Currently, the lack of novel, validated cancer targets continues to severely limit the emergence of new therapeutics. Among the molecular targets critical in the pathogenesis of different types of cancers, protein-protein interactions (PPIs) play a very important role. Our work aims to design and develop PPIs small molecular weight antagonists that act on key apoptosis regulators, with an emphasis on melanoma and Sézary Syndrome.
One of the key factors that contribute to malignant cells longevity is the misbalance between pro-survival and pro-death molecules. Furthermore, evasion of apoptosis, one of the hallmarks of cancer, results in cancer cells resistance to current treatment approaches. The unraveling of the molecular mechanisms controlling apoptosis in cancer will therefore provide: i) a better understanding of the studied diseases and ii) a rationale for the development of specific drugs that modulate pathological apoptosis. To identify new molecules implicated in the apoptotic signaling pathway, we are combining, in collaboration with Hybrigenics S.A. (Paris) and Les Laboratoires Servier (Neuilly-sur-Seine), a large-scale yeast two-hybrid-based protein-protein interaction mapping with a systematic functional validation in mammalian cells and animal models. Our strategy relies on reiterative screens, starting from proteins with known functions in apoptosis and then repeating the screens using the most significant preys as baits to explore the protein network around the selected candidates and to confirm the interaction. Biological validation of the identified targets will allow us to establish a complete protein-protein interaction map (“interactome”) of the apoptosis pathway and identify novel protein-protein interactions as targets for pharmacological intervention. The functional validation of each target includes extensive bibliographic database analysis, monitoring of the endogenous mRNA levels in normal and cancer cells, co-immunoprecipitation and/or co-localization with the bait, in vivo and in vitro protein-protein interaction assays, apoptosis assays following RNA interference-mediated knockdown or expression of dominant negative/positives mutants. These different interference technologies and read-out assays are developed to assign functions to as many proteins as possible. Once a pertinent binary PPI is detected, the next step consists of a quantitative characterization of the interaction, including its structural, biochemical and biophysical parameters. These include locating the binding sites on the surfaces of both proteins, detecting the residues that mediate the interaction and their differential contribution to the interaction, measuring the affinity of the interaction and studying the biological role of the interaction in cells. Once identified and selected, the targets are used to rationally design inhibitory peptides or peptide mimetics based on the sequences that mediate PPIs in the proteins. These peptides are expected to mask a critical part of the binding surface and therefore act as competitive inhibitors of the selected protein-protein interaction. The designed peptides are then conjugate to short, arginine-rich peptide carriers (cell-penetrating peptides, or CPPs) already described or developed in the laboratory in order to allow them to cross biological membranes.
This approach has enabled us the design and patenting of a series of short peptides that are effective as specific inhibitors of apoptosis regulatory complexes identified from yeast two-hybrid screens. These peptides are useful pharmacological tools in cell culture and inhibit tumor growth in vivo in human melanoma xenograft models, BRAF wild type and V600E mutant, with limited to nonexistent off-target toxicity. Moreover, our data indicate that the developed peptides exhibit selective cytotoxicity towards Sézary patients’ T cell clone both ex vivo and in vivo. Our goal will be now to investigate their use as therapeutics in humans.
Boosting the anti-tumor response: the power of NK cells
Project managers: A. Bensussan, A. Marie-Cardine
NK cells are the main cellular actors of innate immune responses, a process dedicated to the elimination of abnormal cells (e.g. virally infected or transformed cells). NK cell killing functions being inhibited in the tumor microenvironment, we aim to develop antibodies against the activating NK cell receptor CD160-TM to promote or amplify an NK celldependent cytotoxicity towards tumor cells.
CD160 has been initially identified as a GPI-anchored MHC-class I activating receptor mainly expressed on peripheral blood NK cells. Its ligands were identified as MHC-class I molecules and HVEM. We additionally reported the identification of a CD160 transmembrane isoform (CD160-TM) resulting from the alternative splicing of CD160 gene. We further established that CD160-TM surface expression is highly restricted to NK cells and is activation-dependent. Unlike CD160-GPI, CD160-TM interacts with MHC-class I molecules but not HVEM. In addition, it was provided evidences that CD160-TM represents a novel activating receptor, its engagement amplifying the NK cells’ responses. Accordingly antibodies that bind to the CD160 transmembrane isoform without binding to the CD160 GPI-anchored isoform can thus be suitable for amplifying NK cell activation and therefore NK cell cytotoxic functions. Beside its expression on normal activated NK cells, an expression of CD160-TM was evidenced on NK cell lines derived from patients presenting NK leukemia or NKT lymphoma, a cutaneous lymphoma with aggressive progression and poor prognosis. We therefore anticipated that CD160-TM might represent a valuable antigen to target malignant NK cells and promote their efficient and specific elimination. Altogether, these data suggested that a specific anti-CD160TM antibody might be considered as a useful tool to: i) amplify the cytotoxic activity of activated NK cells in a tumor environment, ii) drive an anti-tumor activity by targeting CD160-TM-expressing tumor cells and iii) inhibit deleterious cytokines production in identified pathologies. A fully human antibody specific for CD160-TM has been recently selected in our laboratory, in collaboration with the Biotech company Alderaan Biotechnology. Our next steps in validating its use as an immuno-therapeutic tool will be to establish its safety and modes of action as a direct amplifier of NK cell functions, a catalyzer for tumor NK cell targeting (in combination with ADCC-promoting antibodies) or a direct inducer of ADCC towards CD160-TM-expressing malignant cells.
NEWS
Immunity to commensal skin fungi promotes psoriasiform skin inflammation
Charlotte Hurabielle, Verena M. Linka, Nicolas Bouladouxa, Seong-Ji Hana, Eric Dean Merrilla, Yaima L. Lightfootf, Nickie Setof, Christopher K. E. Bleckh, Margery Smelkinsoni, Oliver J. Harrisona, Jonathan L. Linehana, Samira Tamoutounoura, Michail S. Lionakisl, Mariana J. Kaplanf, Saeko Nakajimaa, and Yasmine Belkaida
PNAS, July 2020
Download the news
Principal Investigators
- Anne MARIE-CARDINE, DR2, CNRS
Tél. : 01 53 72 20 78 - Armand BENSUSSAN, DRCE INSERM
Tél. : 01 53 72 20 50 - Nicolas DUMAZ, DR2, INSERM
Tél. : 01 53 72 20 85 - Jean-Luc POYET, DR2, INSERM
Tél. : 01 53 72 20 50 - Samia MOURAH, PU-PH, APHP/Université de Paris
Tél. : 01 42 49 48 85 - Céleste LEBBE, PU-PH, APHP/Université de Paris
- Martine BAGOT, PU-PHEX, APHP/Université de Paris
- Nicole BASSET-SEGUIN, PU-PH, APHP/Université de Paris
- Maxime BATTISTELLA, PU-PH, APHP/Université de Paris

Latest publications
Efficient Therapeutic Delivery by a Novel Cell-Penetrating Peptide Derived from Acinus
Onco-dermatology and therapies, Cancers Jul 10, 2020
Immunity to commensal skin fungi promotes psoriasiform skin inflammation
Onco-dermatology and therapies, PNAS Jul 1, 2020
Expansion of Circulating CD49b+LAG3+ Type 1 Regulatory T Cells in Human Chronic Graft-Versus-Host Disease
Onco-dermatology and therapies, J Invest Dermatol. May 16, 2020


