RESEARCH TEAMS
PATHOPHYSIOLOGY OF BREAST CANCER
Our multidisciplinary team mainly focuses on the physiology and treatments of aggressive subgroups of breast cancers (i.e. triple negative breast cancer (TNBC) and molecular apocrine breast cancer (MABC)), with emphasis on the signaling pathways which impact their aggressiveness. These studies may afford novel and significant designs for anti-tumor therapies.
Breast cancers are highly heterogeneous diseases. Some subgroups of tumors are not biologically well decrypted and consequently do not benefit from optimal therapeutic options. These clinical situations underline the necessity to find innovative therapeutic approaches for these aggressive cancers. To achieve this goal, our multidisciplinary team, composed of basic scientists, clinicians, nuclear physician, pathologists, geneticists and molecular biologists, work on a better characterization of these subgroups, by deciphering the critical signaling pathways regulating the aggressiveness of these breast cancers, to define new therapeutic targets.

Our scientific and medical approaches are organized according to the following 4 complementary topics:
1- NFAT transcription factors and cancer
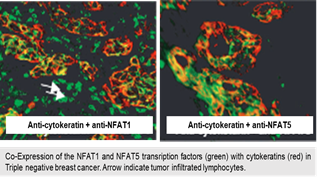
Among these new therapeutic targets, work in our team focuses on the NFAT transcription factors family (NFAT 1-5) in breast cancers. We have demonstrated that NFAT1 (NFATC2) and NFAT5 are expressed and functionally active in TNBC to enhance breast cancer cell invasion and metastasis (Jauliac, Nature Cell Biology 2002 ; Yoeli-Lerner, Mol. Cell 2005 ; German, Oncogene 2012 ; Gaudineau, J. Cell Science 2012).
Moreover, we discovered, in estrogen receptor expressing (ER+), poorly aggressive breast cancers, the specific expression of NFAT3 (NFATc4), with anti-invasive and anti-metastatic functions (Fougère, Oncogene 2010). We already have ongoing studies on large annotated cohorts of patients to explore the expression of the NFATs members in different subgroups of breast carcinoma including TNBC and MABC. We aimed to emphasize the inhibitory function of the NFAT3 isotype to develop potential new therapeutic and prognostic tools. Indeed, we recently demonstrated that extracellular vesicles produced by NFAT3-expressing cells could be a new anti-tumoral tool to tackle cancer development and metastases dissemination (Cardoso Bueno de Camargo, Scientific Reports 2020).
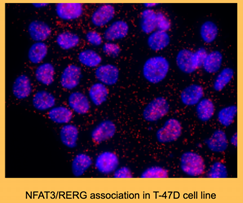 We showed that depending on the isotype NFAT, the function on breast cancer cell invasion was completely opposite. Indeed, we demonstrated that NFAT1 and NFAT5 were pro-invasive (Jauliac, Nature Cell Biology 2002; Yoeli-Lerner, Mol. Cell 2005; German, Oncogene 2012; Gaudineau, J. Cell Science 2012) and NFAT3 was anti-invasive (Fougère, Oncogene 2010). The genes regulation of the NFAT isotypes is specific to each NFAT despite a same highly conserved DNA binding domain. To elucidate these opposing functional effects, we made the hypothesis that specific regions of the different NFAT isotypes might recruit specific proteic partners to ensure their specificity of function. These different proteic partners would then be recruited on sparsely conserved domains specific to each isotype. We have already identified RERG as a functional partner of NFAT3 and showed that this complex is associated with the absence on axillary lymph nodes colonization (Coillard et al., , Frontiers in Oncology 2022).
We showed that depending on the isotype NFAT, the function on breast cancer cell invasion was completely opposite. Indeed, we demonstrated that NFAT1 and NFAT5 were pro-invasive (Jauliac, Nature Cell Biology 2002; Yoeli-Lerner, Mol. Cell 2005; German, Oncogene 2012; Gaudineau, J. Cell Science 2012) and NFAT3 was anti-invasive (Fougère, Oncogene 2010). The genes regulation of the NFAT isotypes is specific to each NFAT despite a same highly conserved DNA binding domain. To elucidate these opposing functional effects, we made the hypothesis that specific regions of the different NFAT isotypes might recruit specific proteic partners to ensure their specificity of function. These different proteic partners would then be recruited on sparsely conserved domains specific to each isotype. We have already identified RERG as a functional partner of NFAT3 and showed that this complex is associated with the absence on axillary lymph nodes colonization (Coillard et al., , Frontiers in Oncology 2022).
 2- Molecular apocrine breast cancer
2- Molecular apocrine breast cancer
Described for the first time in 2005, the molecular apocrine breast cancer subgroup (MABCs) is another aggressive breast cancer that do not benefit from precision medicine and need treatment improvement. We take part of the French breast cancer consortium, that refined the breast cancer taxonomy (Guedj, Oncogene 2012) and individualized the MABCS, concerning up to 10% of breast cancer patients. MABCs are defined by an homogeneous molecular signature of activated Androgen Receptor (AR) pathway in ER-/PR- context with HER-2 negative (corresponding to the LAR for luminal androgen receptor subgroup defined by Lehmann, B. et al) or positive status (Lehmann-Che Breast Cancer Res 2013)
In this context, proof of concept was given with different anti-androgen molecules but therapeutic effects remain modest and optimization is necessary. To go further, we have identified and characterized MABC cell line models. We are now exploring the androgen receptor signaling to unravel the functional cross-talks between activated AR and growth factors receptors, to better understand the mechanisms of response and resistance to targeted treatments. Our final goal is to identify new molecular targets and new drug combinations for MABC patients.
3- Immune crosstalk in cancer progression
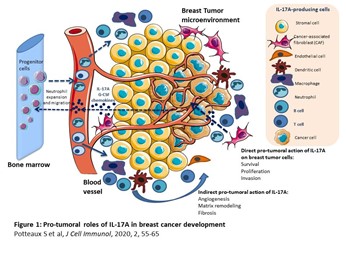
The tumor microenvironment is critical for the development of breast cancer but also needs to be considered for an eventual immunotherapy. Recruitment of immune cells to the tumor and specifically those of the myeloid lineage is a constant process fueled by the continuous secretion of inflammatory cytokines by both malignant, stromal and immune cells. Among these cytokines, we want to elucidate the potential role of IL-17 cytokine members in these cells recruitment and their putative role in the development of immunosuppressive tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs). Our goal is to evaluate the benefit of blocking IL-17 cytokines members to potentiate already used immunotherapy in breast cancer (Potteaux, J Cell Immunol, 2020).
4- Translational research
The translational part of our work deals with the identification of early markers of response and resistance to treatment in advanced breast cancers. We have shown the usefulness of dynamic exploration of tumor metabolism by 18F-FDG uptake during neoadjuvant treatment to early evaluate the response to treatment (Groheux, Eur J Cancer 2014). We also demonstrate that associating molecular tumor biomarkers to 18F-FDG uptake at baseline and 2nd cycle can optimize the identification of patients that do not respond to neoadjuvant therapy and need early modifications of treatment. We now evaluate the input of artificial intelligence (AI) tools to combine all the identified biomarkers to a pertinent predictive algorithm. Moreover, we use our expertise in nuclear medicine to explore other PET/SCAN imaging biomarkers to improve clinical follow up.
Especially, we are focusing on specific radioactive tracers able to imaging the functionality of the androgen receptor in MABC.
PROJECTS
NFAT transcription factors and cancer
Project managers: Sébastien Jauliac , CRCN-Inserm, publications on ORCID
We study the role of NFAT transcription factors in the regulation of the invasive capacities of breast carcinoma, contributing to the formation of metastases. The goals of our studies are to reveal the mechanisms downstream NFAT factors involved in cancer to be able to provide new therapeutic targets and tools to prevent or cure breast cancer metastases.
Cancer is one of the leading causes of morbidity and mortality worldwide with an expected increasing number of new cases in the next decades. Regardless of the primary site, the leading cause of death is the formation of metastases. Despite the development of new strategies, efficient treatments are still needed especially for so-called “aggressive” cancers with a high rate of metastases formation (i.e. triple negative breast cancer and pancreatic cancer). Considering the metastatic players in breast cancer biology, we have previously shown the role of NFAT transcription factors in the dissemination of metastases. We demonstrated that the transcription factor NFAT1 (NFATc2) and NFAT5 exert a pro-invasive function (Jauliac, Nature Cell Biology 2002; Yoeli-Lerner, Mol. Cell 2005; German, Oncogene 2012; Gaudineau, J. Cell Science 2012), whereas NFAT3 (NFATc4) has anti-invasive properties limiting the aggressiveness of primary NFAT3-expressing luminal breast cancer cells (Fougère, Oncogene 2010). Since then, several publications have highlighted the critical role of NFAT transcription factors in tumorigenesis in many other cancers (melanoma, pancreas and lung). Identifying effective treatments of “aggressive cancers” with high rate of metastases formation (i.e. triple negative breast cancer (TNBC) and pancreatic cancer) is the urgent challenge of the next decade. Indeed, TNBC remains the breast cancer subgroup with the least benefit from targeted therapies. Therefore, the early-stage TNBC patients are mainly treated with combinations of taxane and anthracycline chemotherapy with severe side effects and unfortunately often a recurrence of the metastases in the first 3 years after surgery. These therapeutic situations underline the urgent necessity of finding innovative therapeutic approaches to treat these aggressive cancers. To fulfil this necessity, based on our previous work on the NFAT transcription factors in breast cancer, we aimed to divert the inhibitory function of the NFAT3 isotype to develop potential new therapeutic and prognostic tools that will help to tackle cancer development and/or metastatic propension. To this our project is developed in 2 axes.
Developing therapeutic tools using the inhibitory function of NFAT3: Inhibitory extracellular vesicles.
We demonstrated that we were able to produce inhibitory extracellular vesicles (EV) derived from luminal breast cancer cell lines expressing endogenously NFAT3. We have shown in vitro that these inhibitory EV were fully competent to impair TNBC cell lines invasion but interestingly also the invasion of other highly aggressive cancers (melanoma, glioblastoma and pancreatic cancer). Critically, we expanded these in vitro results in an in vivo orthotopic TNBC mice model where these inhibitory EVs were qualified to inhibit metastases arising and tumour growth. Moreover, we highlight that these EV were efficient to block the growth of a pre-established tumour. Mechanistically, we prove that expression of a functional NFAT3 in the EV-producing cells was necessary for EV to exert both in vitro and in vivo their inhibitory functions. With the idea in minds to develop efficient tools to treat aggressive cancers, we established the proof of concept that over-expressing a constitutively active form of NFAT3 in the EV-producing cells was a suitable way to increase the inhibitory function of the resulting EV in vitro and in vivo (Cardoso Bueno de Camargo, Scientific Reports 2020). These results have been patented with the SATT-INNOV Île-de-France (ERGANEO).
- Work in progress
We currently work on the mechanisms used by these inhibitory extracellular vesicles to impair tumor growth and metastasis spreading. We collaborate with a biophysicists team to generate potential therapeutic extracellular vesicles to treat aggressive cancers.
Deciphering the mechanisms of action of the NFAT3 inhibitory function.
We showed that depending on the isotype NFAT, the function on breast cancer cell invasion was completely opposite. Indeed, we demonstrated that NFAT1 and NFAT5 were pro-invasive (Jauliac, Nature Cell Biology 2002; Yoeli-Lerner, Mol. Cell 2005; German, Oncogene 2012; Gaudineau, J. Cell Science 2012) and NFAT3 was anti-invasive (Fougère, Oncogene 2010). The genes regulation of the NFAT isotypes is specific to each NFAT despite a same highly conserved DNA binding domain. To elucidate these opposing functional effects we made the hypothesis that specific regions of the different NFAT isotypes might recruit specific proteic partners to ensure their specificity of function. These different proteic partners would then be recruited on sparsely conserved domains specific to each isotype.
- Work in progress
We currently work on the identification of potential partners used by NFAT3 to exert its anti-tumoral functions.
Molecular apocrine breast cancer
Project managers:
- Morgane Le Bras, MCU Université de Paris, inserm, u-paris, publications on ORCID
- Jacqueline Lehmann-Che, MCU-PH Université de Paris
We investigate the molecular apocrine breast cancer (MABC) subgroup, defined by the absence of estrogen receptor expression (ER-), with concomitant androgen receptor overexpression (AR) and activated signature of androgen signaling. This subgroup of breast cancer lacks specific targeted therapy. Therefore the goals of our studies are to elucidate the molecular basis of the aggressiveness of the MABC to propose new therapeutic targets.
The molecular apocrine subgroup of breast cancer (MABC) that represents up to 10% of breast cancers do not really benefit from targeted therapy so far. This subgroup is defined by a transcriptional signature that could be resumed by absence of estrogen receptor (ER-) expression, but overexpression of pioneer factor FOXA1, androgen receptor (AR) and the consecutive signaling with AR-related genes. Whereas the roles of this activated androgen signaling are well understood in prostate cancer, its involvement in breast cancer still needs deeper analysis. However, AR could be a potential target of treatment. For example, on MABC cell lines, targeting AR was demonstrated to be efficient to block proliferation and clinical trials in the MABC context reveal the utility of AR targeting, but this needs further improvements. Moreover, MABC constitutes an aggressive breast cancer subgroup, with HER2 amplification in 50% of cases, that our lab and others contributed to characterize (Guedj, Oncogene 2012) . Then, depending on their HER2 status, MABC may exert distinct molecular signaling, which must be specified, to identify putative therapeutic targets. In this context, the main objectives are developed in 2 axes.
Deciphering the molecular mechanisms implied in MABC aggressiveness
This part of the project aims to extend the exploration of the intracellular signaling of this MABC subgroup and the possibility to block corresponding pathways and to even go further in genomic analysis of this specific sub-group. These questions are addressed using specific human molecular apocrine cell lines available and characterized in the lab and molecular (Si-ShRNA) and pharmacological modulation of AR and other targets (HER2 , PI3K). Our results are challenged in vivo, using 2D and 3D-cell lines models, cell lines xenografts or patient derived xenografts (PDXs). We also explored implications of specific miRNA that could contribute to MABCs biology.
Altogether, these combined approaches held in collaboration with colleagues from American University of Beirut (AUB, Lebanon) and other teams in the HIPI Unit will lead to a better understanding of the pathophysiology of MABC, from a basal mechanistic level (genomic alterations and consequent functional signaling pathways) to therapeutic initiatives, targeting this poor-prognosis breast cancer subgroup.
Work in progress
We currently work on the molecular signaling, sensitivity and response to treatment by androgen or anti-androgen molecules is deeply analyzed. In addition, we take advantage of our facilitated access to specific inhibitors and new molecules, to test the relative contribution of already known targets in breast carcinogenesis, such as tyrosine kinase receptor family members (HER family), AR variants and their inter-connexions and downstream partners. Moreover, based on our expertise in controlled inhibitory extracellular vesicles by NFAT3-expressing cells production and purification (Cardoso Bueno de Camargo, Scientific Reports 2020), they will be used either alone or in combination with AR or signaling blockade, as an innovative treatment opportunity.
Evaluation of the impact of the immune infiltrate in MABC treatment response
The tumor microenvironment is critical in the tumorigenesis process and in the treatment effectiveness. Therefore we feel critical to better characterize and understand the implication of the MABC tumoral microenvironment.
- Work in progress
We currently work on characterization of the immune infiltrate present in the MABC microenvironment in silico. Next, we will explore this microenvironment on well defined MABCS cohorts. This characterization is necessary to evaluate the impact of the immune environments on the efficacy of different treatments, especially the possibility of immunomodulation in molecular aprocine breast cancer .
Immune crosstalk in cancer progression
Project managers: Stéphane Potteaux , CRCN-Inserm, publications on ORCID
Myeloid cells and cytokines interplay in the tumor-microenvironment (TME) to promote tumor growth and immune evasion. The IL-17 family members, which are strong regulators of the inflammatory response, remain poorly explored in MABC and TABC (Potteaux, J Cell Immunol, 2020). Our work aims to characterize the contribution of myeloid cells in the immunological field of MABC and TNBC and to envisage the IL-17 members as potential new therapeutic targes.
While a very substantial progress has been made in recent years in immunotherapy of human cancers, the mechanisms of immune escape and immune resistance to cancer immunotherapy are not fully understood. Our objectives are to characterize the immunological features of MABC and TNBC and the role of the tolerogenic cytokine IL-17 in order to propose new targets for cancer immunotherapy. Our main objectives are depicted in 2 axes.
Relationship between IL-17 and the tumor-recruited cells
Tumor-recruited myeloid cells, including tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) are critical in cancer development, immune escape and acquisition of resistance to therapies. Recruitment of myeloid cells to the tumor is a constant process fueled by the continuous secretion of inflammatory cytokines by both malignant, stromal and immune cells.
- Work in progress
We evaluate how the tolerogenic cytokine milieu, in particular the IL-17 cytokine members, in the tumor microenvironment conditionate myeloid cell mobilization, recruitment and differentiate into immunosuppressive TAMs and MDSCs.
Is antagonism of IL-17 a possible new treatment to cure breast cancer?
Once we will have established the role of the IL-17 cytokine members in the modulation of the tumoral microenvironment, we will explore the opportunity to control its expression to improve the therapeutic response.
- Work in progress
We evaluate the different possibilities to antagonize IL-17 cytokines members to potentially use these tools to modify TAMs and MDSCs recruitment in the tumoral microenvironment of MABC and TNBC
Translational research
Project managers :
- Jacqueline Lehmann-Che, PU-PH Université de Paris, My publications on ORCID
- Luis Teixeira, PU-PH Université de Paris, My publications on ORCID
- David Groheux, PH APHAP, My publications on ORCID
In locally advanced breast cancer, neoadjuvant chemotherapy (NAC) gave the opportunity to evaluate the efficacy of treatment with pathological complete response (pCR) on surgery. It’s clear now that pCR is an excellent surrogate marker of survival and prognosis, especially in TNBC and HER2 breast carcinoma, however, the pCR is evaluated late, after 6 or 8 cycles of treatment.
The aim of our group is to identify early markers of response to neoadjuvant therapies, to avoid useless treatment and to allow early modification of treatment. Our leader expertise in nuclear medicine, allow us to combine metabolic imaging (18-FDG uptake) and tumor genomics to propose pertinent predictive tools, now optimized by radiomics and AI input.
Stages II-III TNBCs retain a poor outcome despite adequate treatment. NAC is a major weapon against this disease and it’s well known that patients with pathological complete response (pCR) after NAC have a good prognosis whereas non-responding patients have a 25-40% risk of distant relapse at 5 years. pCR is therefore currently considered as a major goal in TNBC but need to wait the end of the NAC course for evaluation at surgery.
So, an early prediction of residual disease, during the NAC course, could allow early treatment modification to increase the pCR rate and subsequent patients’ survival.
The goal of our team is to define the best biomarkers allowing this early evaluation of the response to treatment.
In the last years, we have worked to improve molecular imaging (PET) criteria, especially in TNBC. We have shown that early evaluation with PET allow to identify TNBC patients at very low probability (<10%) of achieving pCR and high risk of recurrence (Groheux D, European Journal of Cancer, 2014; Groheux, Eur J Nucl Med Mol Imaging,2018). We also demonstrated that crucial genetic alterations, like the TP53 status of the tumor could influence the response to chemotherapy. Moreover, other markers are described to be predictive of response as TILS count or the homologous recombination deficiency in response to platinum-based chemotherapy.
The main objective of our work is now to propose combined predictive tools (molecular imaging and molecular tumor biomarkers) allowing early treatment modification after beginning of chemotherapy in a bad prognosis but highly chemosensitive population of TNBC. To date, we combine radiomics and genomics to continue to optimize the prediction algorithm, in collaboration with SOPHiA GENETICS.
We expect that this strategy will improve the outcome of TNBC patients (with chemotherapy intensification if necessary), stop ineffective chemotherapy early and avoid unnecessary side effects.
Principal Investigators
- Jacqueline Lehmann-Che, PU-PH
Tél. : 01 42 49 98 58 - Sébastien Jauliac, CRCN
- Morgane Le Bras, MCU
- Stéphane Potteaux, CRCN
- Luis Teixeira, PU-PH
- Philippe Bertheau, PU-PH
- Marc Espié, MCU-PH
- David Groheux, PH
- Odile Cohen-Haguenauer, MCU-PH