RESEARCH TEAMS
HLA-G IMMUNE CHECKPOINT IN ONCOLOGY AND TRANSPLANTATION
Our team has expertise in immune tolerance mechanisms, with a special interest in those mediated by the checkpoint molecule HLA-G in oncology and transplantation. Starting from our initial demonstration of the fundamental role of HLA-G in maternal-fetal tolerance, a perfect physiological example of successful tolerance of an allogenic graft, we then transposed our observations to solid organ transplantation with the rationale that HLA-G contributes to allograft acceptance. In parallel, we developed studies in oncology based on the deleterious effects of immune tolerance with the role of HLA-G acting as an immune checkpoint and allowing tumor escape from the antitumor immune responses.
Our team (CEA-SRHI Service de Recherches en Hémato-Immunologie) was created in 1991 at Saint-Louis Hospital (AP-HP) in order to benefit from close links with the university hospital and clinical expertise in cancerology and transplantation. The mission of our team is to study immune tolerance mechanisms of tissue grafts, as well as tumor immune escape from host surveillance. Our team has conducted research on HLA-G, a non-classical HLA class I molecule, and its function since 1992. Our team personnel is composed of French university professors/hospital practitioners, foreign professors, researchers, pharmacy and medical residents, as well as graduates from the Grandes Ecoles in France.
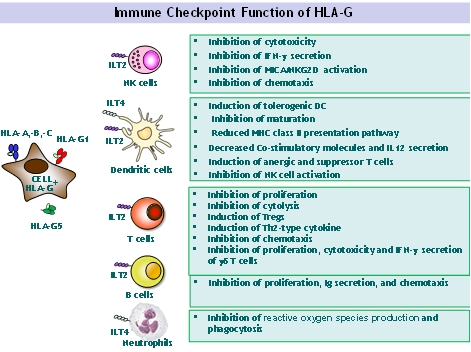
In the 1990s, we were the first to demonstrate the immune tolerogenic function of the HLA-G molecule and its fundamental role in maternal-fetal tolerance. Indeed, HLA-G expression by the fertilized oocyte is essential to embryo implantation and proper development. Because maternal-fetal tolerance is a unique example of successful tolerance of allogeneic tissues, we hypothesized that HLA-G may also induce a state of tolerance in transplants. Thus, we first demonstrated the expression of soluble and membrane-bound HLA-G by allografts, and we showed that HLA-G exerts its tolerognic function by acting at all levels of the allogeneic reaction. Indeed, HLA-G inhibits the cytolytic function of NK cells, the antigen-specific cytolytic function of cytotoxic T lymphocytes (CTLs) and /T cells, the alloproliferative response of CD4+ T cells, and the maturation and function of dendritic cells. We also demonstrated that HLA-G induces regulatory T cells (Tregs) and myeloid suppressive cells (MDSCs). Our work positioned HLA-G as a major immune checkpoint molecule with a broad immunoregulatory functions that affect both innate and adaptive immunity via its interaction with the inhibitory receptors ILT2 and ILT4 (LILRB1/CD85j and LILRB2/CD85d), which are differentially expressed by immune cells. Thus, in contrast to both CTLA-4/B7 and PD1/PD-L1, the ILT/HLA-G checkpoint inhibits all actors, and blocks all stages of an immune response, from APC activation and effector priming, to the function of fully activated CTLs or NK cells.
We also developed studies in cancerology, a pathological context in which immune responses directed against the tumor are compromised. We first demonstrated that even though HLA-G is mostly expressed by fetal cells and not by adult tissues, it was neo-expressed to various degrees by most tumors. In this context, we demonstrated that HLA-G exerted its checkpoint immune inhibitory function and inhibited anti-tumor responses, thus causing immune escape. To develop translational studies in onco-uro-immunology, a translational group under the responsibility of Pr Desgrandchamps was created in 2013 within our team. This clinical group includes teams from Saint-Louis Hospital: Urology (Pr F. Desgrandchamps), Medical Oncology (Pr S. Culine), Radiotherapy (Pr C. Hennequin), Interventional Radiology (Pr E. de Kerviler), and Pathological Anatomy (Dr J. Verine).
Our objective is to continue our research on the immune checkpoint molecule HLA-G in the context of transplantation and cancerology. At the fundamental level, we aim at defining the link between HLA-G and other checkpoints, especially PD1/PDL1, and at understanding mechanisms controlling immune tolerance. Our final purpose is to go into clinical applications at the level of (i) therapy, by promoting tolerance mediated by HLA-G as anti-graft rejection strategy, while breaking down tolerance through anti-HLA-G for antitumor therapy, and (ii) monitoring, by identifying new markers for prognosis and treatment efficacy. In this regard, our team has been organizing the HLA-G international conference and workshop every three years since 1998.
FUNDING
Our laboratory is currently funded by CEA, APHP, Université Paris-Diderot, INSERM, and grants from ANR, INCA/DGOS, Roche Institute, AFRETH, Foncer contre le cancer, Fondation Air Liquide, Vaincre la Mucoviscidose
PROJECTS
OBJECTIVE 1: Position HLA-G as a new checkpoint therapeutic target in tumor immunotherapy
To restore an effective anti-tumor response, blocking checkpoints by mAbs is the currently favored immune-therapeutic strategy. The checkpoint molecule HLA-G is not expressed in most normal adult tissues but is expressed in most cancers. Animal models have shown that HLA-G-mediated tumor immune escape can be abrogated by the use of mAb, and anti-tumor function restored. Our objective is to demonstrate in urologic cancers, that in the event of anti-PD1/PDL1 therapy non-responsiveness, the HLA-G/ILT checkpoint constitutes a better target choice.
To achieve our program, we have brought together scientists and clinicians oncologists, urologists and pathologists from Saint-Louis hospital (Paris) to: (i) analyze the impact of microenvironment on intratumor heterogeneity of HLA-G and PDL1; (ii) define the HLA-G isoforms relevant to the inhibition of anti-tumor lymphocytes; (iii) precisely characterize intratumor ILT2+ CD8+ and CD4+ T cells; and (iv) provide the clinical relevance of the HLA-G/ILT checkpoint in a cohort of urologic patients under anti-PD1/-PDL1 immune therapy. Molecular and cellular immune assays are performed (transcriptomic, cell proliferation, cytotoxicity, cytokine secretion) both ex vivo with patient samples (blood and tumor) and in vivo in murine models. We expect to demonstrate that the HLA-G/ILT checkpoint is a relevant therapeutic target in case of non-responsiveness to anti-PD1/PDL1, and that anti-HLA-G antibodies are efficient as new immunotherapy to be used in coordination with other treatments.
Analysis of the impact of hypoxia, DNA methylation and miRNAs on HLA-G and PDL1 intratumor heterogeneity
We recently showed that PD1/PDL1 and HLA-G/ILT checkpoints are heterogeneously expressed in the various areas of the same renal tumor. In order to analyze the mechanisms involved in such intratumor heterogeneity of HLA-G and PDL1, we chose to perform a selected gene approach by focusing in clear cell renal-cell carcinoma (ccRCC)areas on: i/ the expression of immune checkpoint regulators such as Hypoxia Inducible Factors HIF-1α and HIF-2α; ii/ DNA methylation state of the immune checkpoint genes (5’URR and coding regions; and (iii) microRNAs that regulate HLA-G and PDL1 expression in tumor and plasmas.
Although HLA-G expression is restricted to a few immune privileged tissues in normal conditions, it is very frequent in tumors. We previously investigated the different protagonists in the regulation of tumor HLA-G expression with the aim to find a way of controlling it. We previously described several specific HLA-G regulatory elements, transcription factors and microenvironmental modulators controlling HLA-G expression in tumor cell lines. In particular, we analyzed the induction of HLA-G transcription by hypoxic conditions, a key factor in aggressive tumors. In vitro, we established that upon treatments mimicking hypoxia, Hypoxia Inducible Factor-1 (HIF-1) targets a main Hypoxia Responsive Element (HRE) located in exon 2 of the gene (IMGT nomenclature). A second polymorphic HRE site (-966A-G) located in the 5’ upstream regulatory region (5’URR; 1.4 kb upstream the ATG), was demonstrated to modulate the level of the response. Otherwise, since most cancers are characterized by localized aberrant CpG methylation silencing both tumor-suppressor genes and genes encoding tumor-associated antigens, demethylating treatment is one of the epigenetic therapies used to block tumor progression. We demonstrated that following 5-aza-2’deoxycytidine treatment, HLA-G expression is upregulated in HLA-G-tumor cell lines and is increased in the presence of desferrioxamine (a hypoxia mimicking agent) or INF-β. Regarding intratumor heterogeneity of HLA-G in renal tumors and its co-expression or not with PDL1, the next steps are to investigate i/ the impact of HIF-1α/-2α and DNA methylation of HLA-G and PDL1 in tumor areas; and ii/ the impact of selective miRNAs regulating HLA-G and PDL1 in both tumor areas and plasma from patients.
Characterization of tumor-expressed HLA-G isoforms and receptors
The HLA-G primary transcript is alternatively spliced leading to seven alternative mRNAs able to encode four membrane-bound (HLA-G1 to HLA-G4) and three soluble (HLA-G5-HLA-G7) protein isoforms. Recently, in the context of oncology our team described novel HLA-G spliced transcripts lacking the alpha 1 domain or with a 5’-extended part. In fact, HLA-G isoforms were always found as inhibitory checkpoints. Determining the presence of particular isoforms in tumor is essential to therapeutically target the precise molecule and have insight in the molecular mechanism of action.
Using a molecular approach based on an unbiased strategy that combines transcriptome determination and immunohistochemical labeling, we provided the first extensive portrait of HLA-G in clear cell renal-cell carcinoma. We demonstrated that HLA-G expression brings forth a high level of intra- and intertumor heterogeneity. Moreover, our results generated an inventory of as yet undescribed spliced HLA-G isoforms which includes transcripts that have an extended 5’-region and lack the transmembrane and alpha-1 domains. So far, these isoforms could not be detected by any currently available methods. In the present project, we will compare the function of the recently identified new HLA-G isoforms, i) in vitro by methods routinely used in the laboratory; ii) in vivo, by using xenografts which will allow determining whether the presence of a particular isoform might be beneficial or disadvantageous for the outcome of the tumorigenic cancer cells. For this purpose, we used clear cell renal-cell carcinoma (ccRCC) cell lines to be transduced with lentivirus vectors encoding each isoform.
Demonstration of anti-tumor function of ILT2+ tumor infiltrating CD8+ and CD4+ T cells and their inhibition by HLA-G+ tumors
We previously demonstrated that high ILT2 expression on peripheral blood CD8+ T cells of patients with non-muscle invading bladder cancer was strongly associated with recurrence. We also showed that both CD8+ ILT2+ and CD4+ ILT2+ T cells infiltrate HLA-G+ kidney tumors. The aim of this project is to better characterize the functions of these CD8+ILT2+ and CD4+ ILT2+ T cells, determine if they are of anti-tumor specificity, and demonstrate that their reactivation by anti-HLA-G antibodies leads to tumor regression.
In order to characterize ILT2-expressing T cells, we conducted transcriptomic studies on CD8+ILT2+ T cells vs. autologous CD8+ILT2- T cells, validated the results by flow cytometry, and investigated their function. These investigations allowed us to precisely define the subset of CD8+ T cells expressing ILT2 and demonstrate that they are terminally differentiated cytotoxic T cells. They are found in the peripheral blood of healthy donors and cancer patients and sometimes infiltrate urologic HLA-G-expressing tumors (kidney and bladder). Our data indicate that these cells are sensitive to inhibition by HLA-G but not by other immune checkpoints. This would make them a potential anti-tumor immune subset that could not be reactivated by the currently investigated checkpoint inhibitors, but only by anti-HLA-G/anti-ILT2 antibodies. We aim to extend the validated CD8+ILT2+ findings to the CD4+ T cell subset. This will be done using ex-vivo material (peripheral blood, tumor-infiltrating cells, and tumor cells) obtained from urologic cancer patients. We will focus on in vitro studies, and on an already on-going collaboration with CEA-SEPIA (CEA FAR, IBFJ, JP Deslys) aimed at setting up animal models of human tumor xenotransplant and human immune system reconstituted mice. These studies are important on a basic research standpoint, to better understand the nature and functions of HLA-G-sensitive CD4+ and CD8+ T cells. They are also crucial in the context of our demonstration that therapeutic anti-HLA-G blocking antibodies may be useful as anti-checkpoint immunotherapy.
Demonstration that HLA-G/ILT2 is a relevant checkpoint in therapy-resistant tumor tissues after anti-PD1/PDL1
Our working hypothesis is that HLA-G/ILT2/ILT4 checkpoint is a critical parameter to be considered in designing future immunotherapy strategy in urologic cancer patients to prevent the ineffectiveness of current anti-checkpoint monotherapy, such as anti-PD1 or anti-PDL1, and to customize coordinated immunotherapy. Supporting this hypothesis, we recently showed that the expression of PDL1/PD1 was heterogeneous within the tumor and could coexist with expression of HLA-G/ILT2/ILT4; constituting one explanation for the primary resistance to single-PD1/PDL1 target immunotherapy observed in most patients.
The objective of this project is to define whether HLA-G/ILT2/ILT4 checkpoint may account for therapy resistance in patients treated with anti-PD1/PDL1. To this end, we are conducting a prospective monocentric observational study whose principal aim is the evaluation of the impact of HLA-G expression within the tumor on response to anti-PD1 or anti-PDL1 immunotherapy (as monotherapy or in combination with anti-CTLA4), in patients treated for metastatic or inoperable urologic cancer. This 3-year prospective study is carried out on 75 patients with metastatic renal cell carcinoma and 25 patients with invasive bladder cancer enrolled in clinical trials with anti-PD1 or anti-PDL1 (service of medical oncology directed by Pr. S. Culine). The primary endpoint is objective tumor response assessed using periodic imaging exams interpreted using the iRECIST reference methodology. The secondary objectives of this study are to: (i) evaluate the frequency of PDL1 and HLA-G expression co-occurrence, (ii) evaluate the impact of HLA-G expression in the tumor on progression-free survival and overall patient survival, (iii) search for an association between HLA-G expression in the tumor and plasma soluble HLA-G levels, (iv) evaluate the impact of plasma soluble HLA-G levels on tumor response to immunotherapy, progression-free survival, overall survival and the occurrence of immunological complications under immunotherapy, (v) evaluate the impact of the distribution of circulating lymphocyte subpopulations, characterized by the expression of immune checkpoint receptors (including ILT2 and PD1), on these same parameters.
OBJECTIVE 2: Position HLA-G as a graft stability diagnostic marker and anti-rejection therapeutic tool
Since we first described the expression of HLA-G in the context of heart transplantation in 2000, we have demonstrated that after solid organ transplantation (heart, lung, kidney and liver), HLA-G expression was significantly observed in stable patients compared to patients in rejection. In agreement with that observation, we found decreased anti-HLA Abs, known to be involved in allograft rejection, in HLA-G+ recipients. In this context, HLA-G is present in the transplanted cells (immunohistochemical analysis of biopsy transplant) and in the plasma (where HLA-G soluble forms are measured by ELISA). These studies have allowed HLA-G to be considered as a benchmark for identifying patients with a low risk of rejection, and as a potential anti-rejection therapy (i.e., HLA-G proteins or HLA-G+ cells).
Expression and function of HLA-G/ILT2 and PD1/PDL1 in lung transplantation
HLA-G is well-known as being associated with better acceptance in solid organ transplantation. The aim of this project is to determine in lung transplanted patients whether other HLA-G-related parameters are also associated with better acceptance, and how they relate to other immune checkpoints.
In partnership with the Service de Pneumologie (Pr O. Brugière) at Bichat Hospital (Paris), we described the expression of HLA-G in the bronchial epithelium from clinically stable patients. This expression follows a pattern similar to that previously observed in liver and renal transplants, among recipients unaffected by rejection, in whom renal tubular and hepatic biliary epithelial cells expressed HLA-G. The expression of a tolerance molecule such as HLA-G in these cells would play a protective role with regard to acute and chronic rejection. In lung transplantation, the vital prognosis is clearly associated with the onset of chronic rejection (in the form of bronchiolitis obliterans), which occurs in more than half of lung transplant patients after 3 years. Such chronic rejection results from bronchial epithelial cell (BEC) damages, thought to be orchestrated by T cells primed by APC presenting allo-antigens. Analysis of this cell cross-talk led us to show that BEC play a pivotal immunosuppressive role in T cell alloreactivity through HLA-G, TGF-β, and IL-10 expression. Interestingly, our data showed that the inhibitory properties of BEC are dysregulated in LTx recipients, which could suggest their instrumental role in the initiation of the bronchiolitis obliterans syndrome process.
In this project, we aim to determine in lung transplanted patients (COLT Cohort) whether other HLA-G-related parameters are also associated with better acceptance, and how they relate to other immune checkpoints. We therefore investigate peripheral T cell subsets expressing HLA-G, ILT2, PD1, Tim3, or Lag3, as well as Tregs and tolerogenic DC. The data collected will be compared with other clinico-biological data to determine which of these immunological parameters are associated with a lower incidence of rejection. In parallel, we pursue analysis of the dialogue between BEC and immune cells in the recipient. This part of the project is based on the hypothesis that one of the first levels of modulation is related to the ability of BEC to directly act as APC with regard to T cells. Secondly, BEC could affect the function of immune cells by producing a tolerogenic microenvironment, via the secretion of soluble factors. The ambitious nature of this project resides in analyzing local immunity in the lung transplant through three axis: (i) contribution of HLA-G and other checkpoint molecules in bronchial epithelial cell-mediated immunotolerance, (ii) definition of the mechanism controlling HLA-G expression in grafts, and (iii) effects of anti-HLA alloantibodies on bronchial epithelial cell function. In order to achieve these objectives, we will continue our partnership with INSERM UMR_S 1152 (Marina Pretolani) and Pr Olivier Brugière. Based on these studies, we are engaged in a prospective project on COVID-19 (funded by Air Liquid Foundation).
Validate the Use of Allogenic HLA-G+ MSC from Perinatal Tissues for Cell Therapy
We have previously demonstrated that mesenchymal stem cells derived from bone-marrow express tolerogenic HLA-G contributing to their immunomodulatory properties inhibiting NK cells and T cells. We then validated the use of allogenic bone marrow-derived MSC for bone reconstruction as being partner in a European project. We now want to extend this analysis to MSC from Perinatal Tissues for use in cell Therapy (regenerative medicine and allotransplantation).
Mesenchymal stem cells (MSC) have emerged as alternative sources of stem cells for regenerative medicine because of their multipotency and strong immune-regulatory properties. HLA-G expressed by MSC fulfils an important function since blockade of HLA-G by neutralizing antibodies reverses MSC ability to (i) expand Tregs and (ii) inhibit the alloproliferative T cell response and the cytotoxic function of NK cells. Thus, HLA-G actively contributes to the immunosuppressive properties exerted by MSC with clinical implications in the design of anti-rejection therapeutic strategies. In this regard, we were partners in a European project in which MSC were used in combination with biomaterials to consolidate bone repair in orthopedic and periodontic defects. Our work showed that MSC proved to be hypoimmunogenic and immunosuppressive in allogeneic conditions. Also, a comparative study on the immunosuppressive functions of MSC isolated from extraembryonic tissues (chorion, amnion, Wharton’s jelly and cord blood) shows that regarding their huge availability, the facility to screen a large number of donors and their high proliferative potential, they must be considered as a privileged source for allogeneic cell-based therapies.
Our team is currently involved in an ANR project called “ATYPiCAL” which consists in using MSC from Wharton’s jelly (WJ-MSC), extemporaneously embedded into an Alginate/Hyaluronic Acid (Alg/HA) layer guiding cells to chondrogenic differentiation. An important concern of this project is the use of allogeneic MSC in tissue engineering. Our main work within the project is (i) to characterize the immunomodulatory properties of WJ-MSC during chondrogenic differentiation both in vitro and in vivo studies, and (ii) to check, in vitro, that immune response currently observed in an allogeneic context does not negatively interfere with chondrogenic differentiation. The expected results should allow assessing the feasibility and validating the clinical grade amplification technique as well as obtaining precisely characterized and secured MSC. This project will eventually diversify the Advanced Therapy Medicinal Product (ATMP) field in France implementing an essential skill to therapeutic progress.
Principal Investigators
- Edgardo CAROSELLA, Chef de service CEA, DR CEA
Tél.: +33 (0)1 57 27 67 79 - Nathalie FREISS, Chef de laboratoire CEA, DR CEA
Tél.: +33 (0)1 57 27 68 01 - Joël LEMAOULT, Chef de laboratoire CEA, DR
Tél.: +33 (0)1 57 27 68 02 - Philippe MOREAU, DR
Tél.: +33 (0)1 57 27 67 32 - Diana LE ROUX, DR
Tél.: +33 (0)1 57 27 67 33 - Bela PAPP, CR1 INSERM
Tél.: +33 (0)1 57 27 67 78 - François DESGRANCHAMPS, PU-PH, APHP/Université de Paris
Tél.: +33 (0)1 42 49 96 21 - Stéphane CULINE, PU-PH, APHP/Université de Paris
Tél.: +33 (0)1 42 49 42 47 - Christophe HENNEQUIN, PU-PH, APHP/Université de Paris
Tél.: +33 (0)1 42 49 90 24 - Alexandra MASSON-LECOMTE, MCU-PH, APHP/Université de Paris
Tél.: +33 (0)1 42 49 96 21 - Jérôme VERINE, MCU-PH, APHP/Université de Paris
Tél.: +33 (0)1 42 49 46 88 - Eric De KERVILER, PU-PH, APHP/Université de Paris
Tél.: +33 (0)1 42 49 91 22

Latest publications
HLA-G/LILRBs: A Cancer Immunotherapy Challenge
Jul 1, 2021

Latest news
Alix Jacquier’s thesis, on February 11, 2021
Feb 8, 2021


